WORK EXPERIENCE & DEVELOPED PRODUCTS
I have combination of 3+ years of R&D design consultancy, 4 years at start-ups, and 8 years at Medtronic in various medical device products

about me
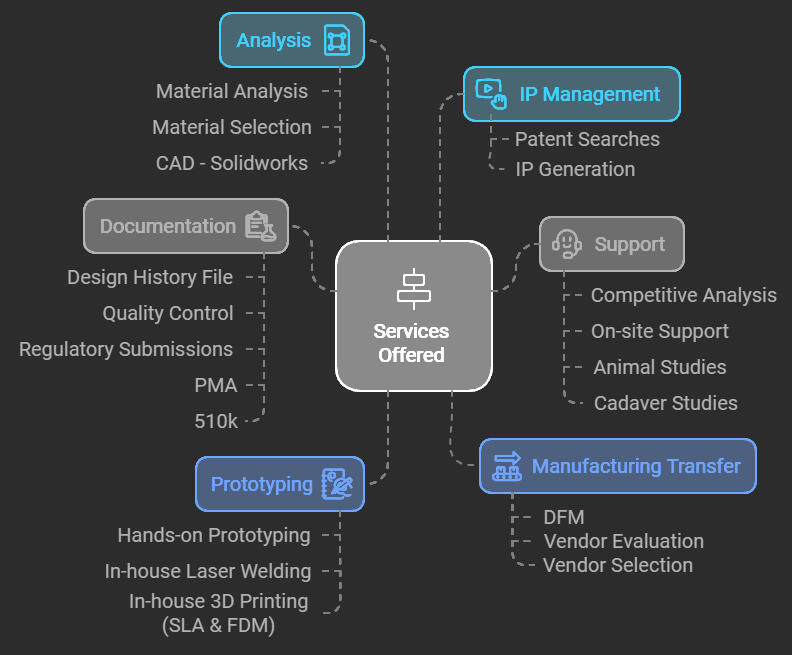
I currently run my own product design consultancy for medical device companies (2021-present) to provide full-scale R&D from development to design verification services.My past experience includes 8 years at Medtronic (transcatheter heart valves and peripheral products) and 4 years at start-ups various companies.
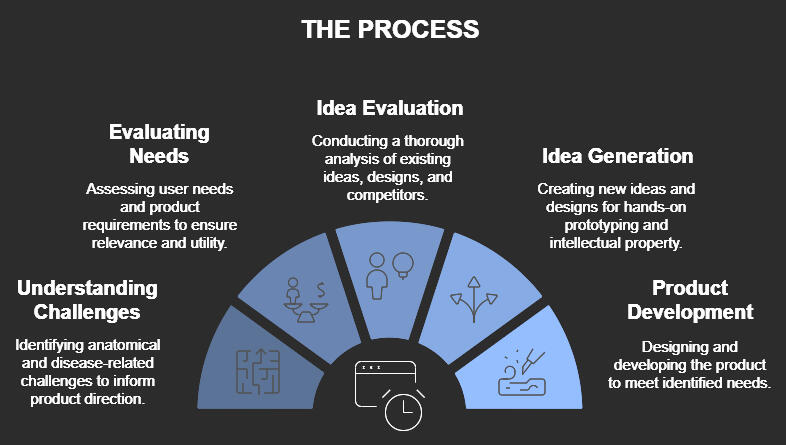
services offered
Support From All Phases:
Feasibility to Commercial Launch
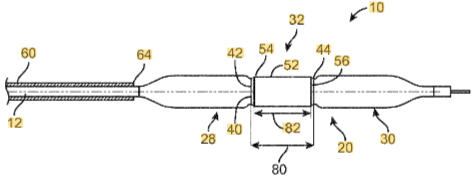
neurovascular experience
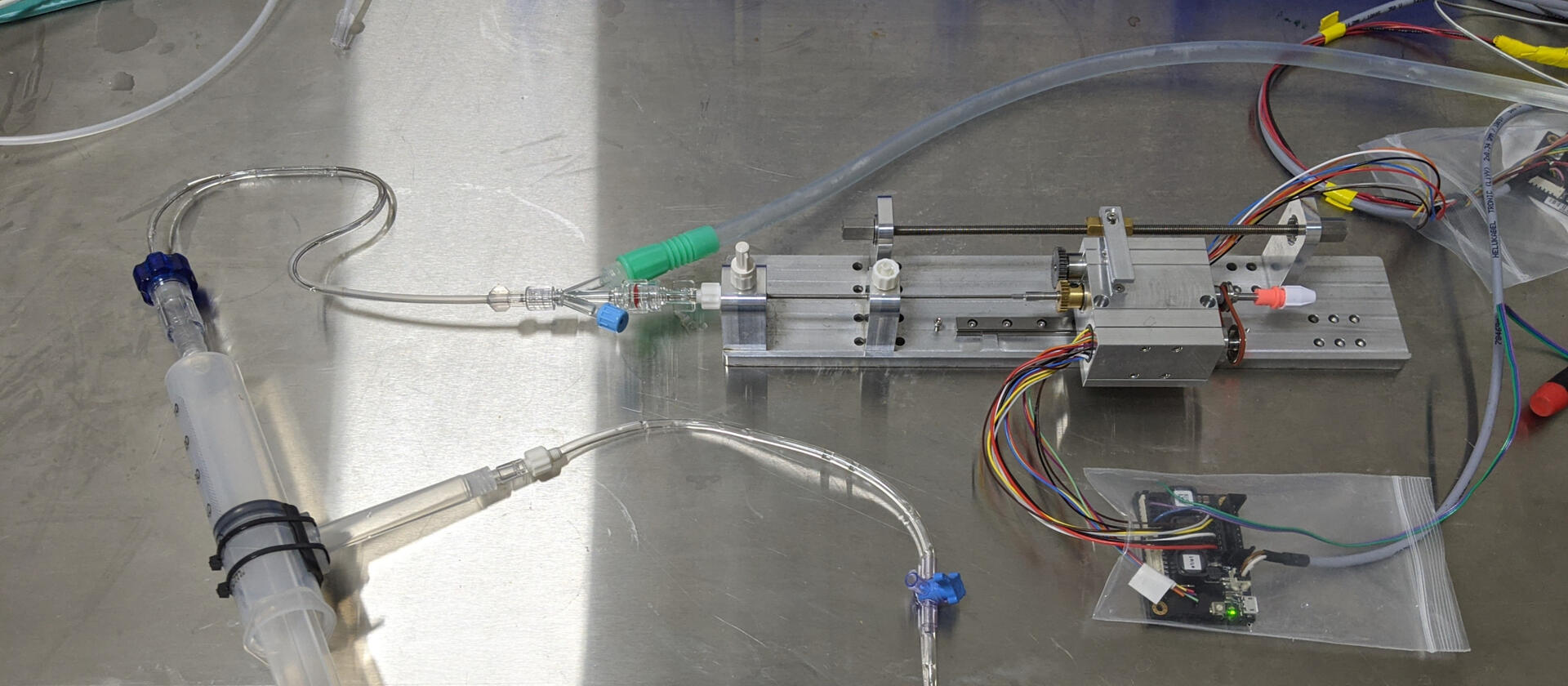
As the lead engineer, I evaluated solutions for ischemic stroke blockage with first pass treatment. I took a concept to prototypes by understanding the morphology of the clot and anatomy challenges to get to it. The initial prototypes worked and showed functionality and a company was started out of the technology.

I led the project to design a mechanical thrombectomy device for the Asian market, this device was a controlled expansion stent retriever design to fully engage clot for removal. This project from idea to concept to working prototype was done in 3 months.
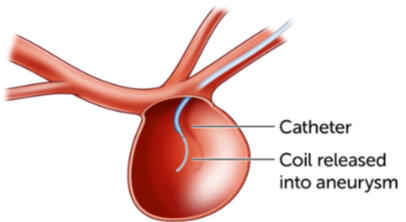
I managed a small team to take the existing microcatheter design and make it smaller and more flexible for drug and coil delivery to treat brain aneurysms. This included managing vendors for the new design and testing as well.
Structural Heart Experience
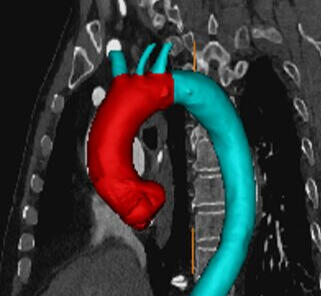
I evaluated solutions for ascending square-ness delivery of stent graft implant while investigation anatomical challenges of horizontal aortic arches. This involve coming up with new concepts and designs, building the prototypes and testing them to provide results. This led to evaluations of deflectable systems for challenging arches and high force deployments.
I worked aortic and trans-femoral delivery systems to prepare for manufacturing in China. This included design for manufacturing improvements, drawings and molded parts. I worked on the improvement of the release mechanism of the delivery system to the implant.
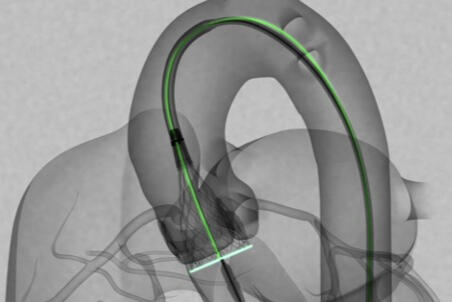
I developed 10+ new delivery system test methods and tested all delivery system components to provided design inputs. I also worked on retractable laser cut designs housing for implant. I also gained experience on the quality system side, learning quality tools like risk analysis, GR&R, and V&V.
Pulmonary experience
As lead delivery system engineer, I was in charge of constructing the catheter delivery system for challenging airways. The design worked with intricacies of navigation of airways and the interaction with the bronchoscope for assistance. This included design of the delivery system, design for manufacturability, and preparing for design verification.
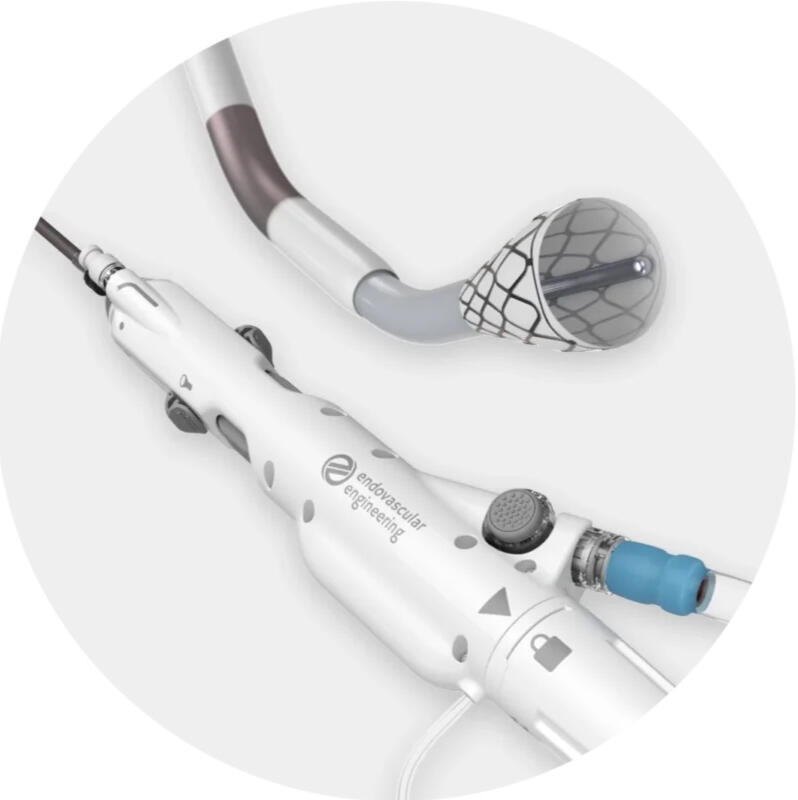
As the lead engineer, I was in charge of the early testing and evaluation of the system that lead to current system in clinical trials today. I was leading the delivery system design from ground up with focus on the funnel and delivery system shafts. I established the catheter design, assembly, and testing of the prototypes ready for design freeze.
Peripheral & DVT Experience
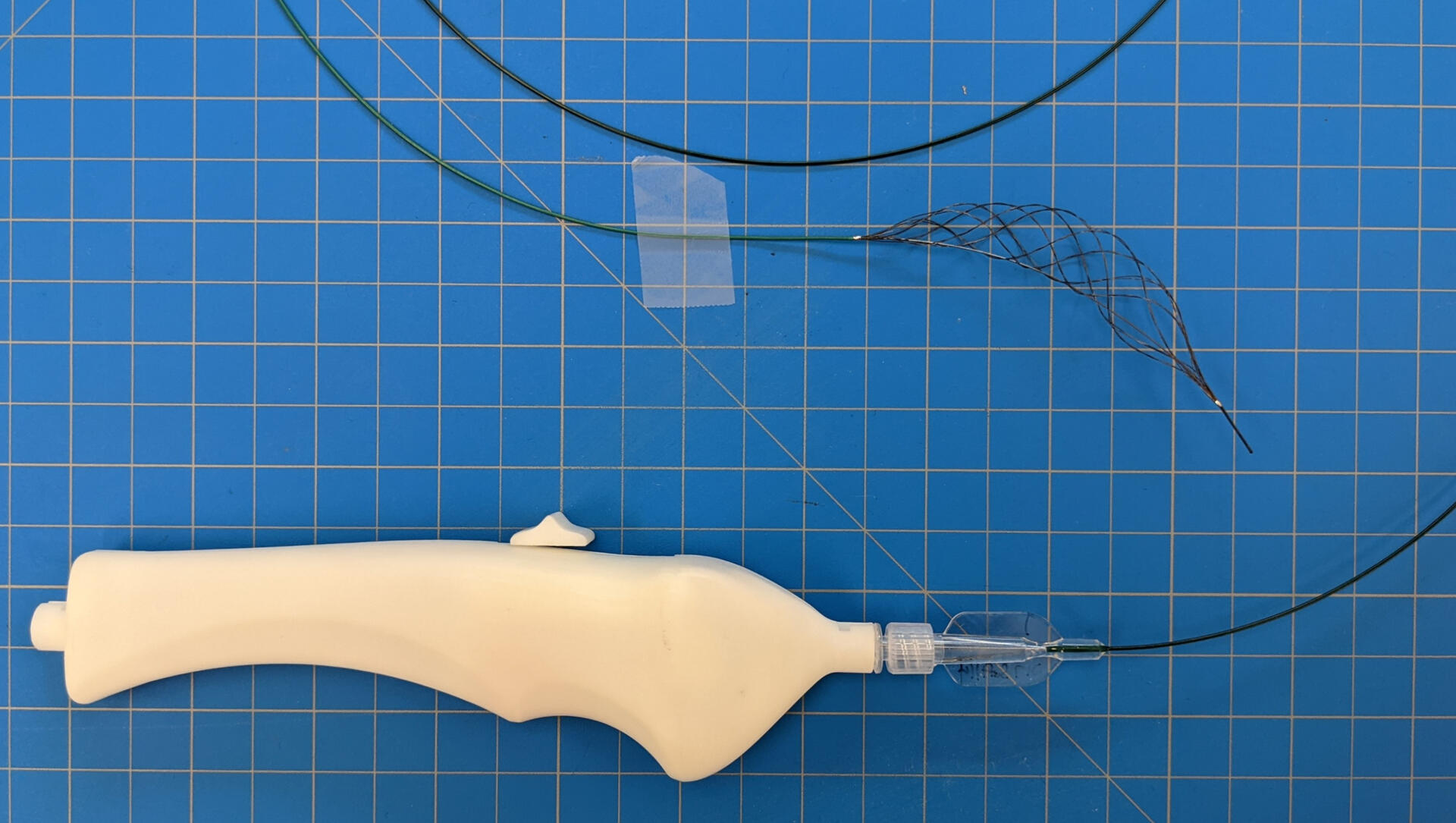
As the project lead, I was in charge of the development of a next gen mechanical thrombectomy stent retriever for peripheral & DVT. This included the design various controlled expansion catheter system prototypes and systems for full engagement of blood clots. This work from idea to concept to working prototypes in 6 months.
As the R&D Manager, I managed the existing design of balloon occlusion microcatheters including manufacturing and testing of the product. I was in charge of running the day to day task of the R&D team. In addition I lead the team to evaluate next gen catheter designs for high pressure drug delivery.
I oversaw the initial product and technology transfer from Europe to in-house facility in the US. I was the R&D core team lead for in-stent restenosis project that was awarded accelerated approval by the FDA. This project also received Medtronic's highest honor. I led a novel research project of modeling calcified arteries to simulate atherectomy and DCB combination.
Other products

As the lead engineer, I am in charge of taking the product through manufacturing and the design of the connection attachment of the Markermesh to the Gomco clamp. I established the manufacturing of both the Markermesh and connection piece to the Gomco clamp. These includes laser cutting process, 3D printing, DFM and injection molding.